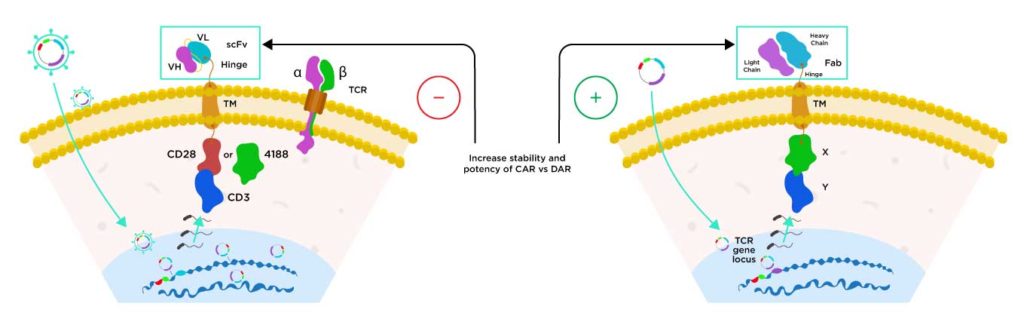
DAR T (Dimeric Antigen Receptor-T Cell)
Sorrento utilizes a proprietary knock-out knock-in (KOKI) technology to modify normal healthy donor derived T cells to genetically engineer them to express the dimeric antigen receptor into T-cell receptor (TCR) alpha chain constant region (TRAC). In this manner, TRAC is knocked out and antigen is knocked into its locus.
The Dimeric Antigen Receptor (DAR) utilizes a Fab instead of the scFv used by traditional Chimeric Antigen Receptor (CAR) T cells. We believe this DAR has been demonstrated in preclinical studies greater specificity, stability and potency.
Current CAR T Cell Technology
Next-Gen Dimeric Antigen Receptor (DAR) Technology