A Fully Intergrated Clinical Stage ADC Company
About Oqory
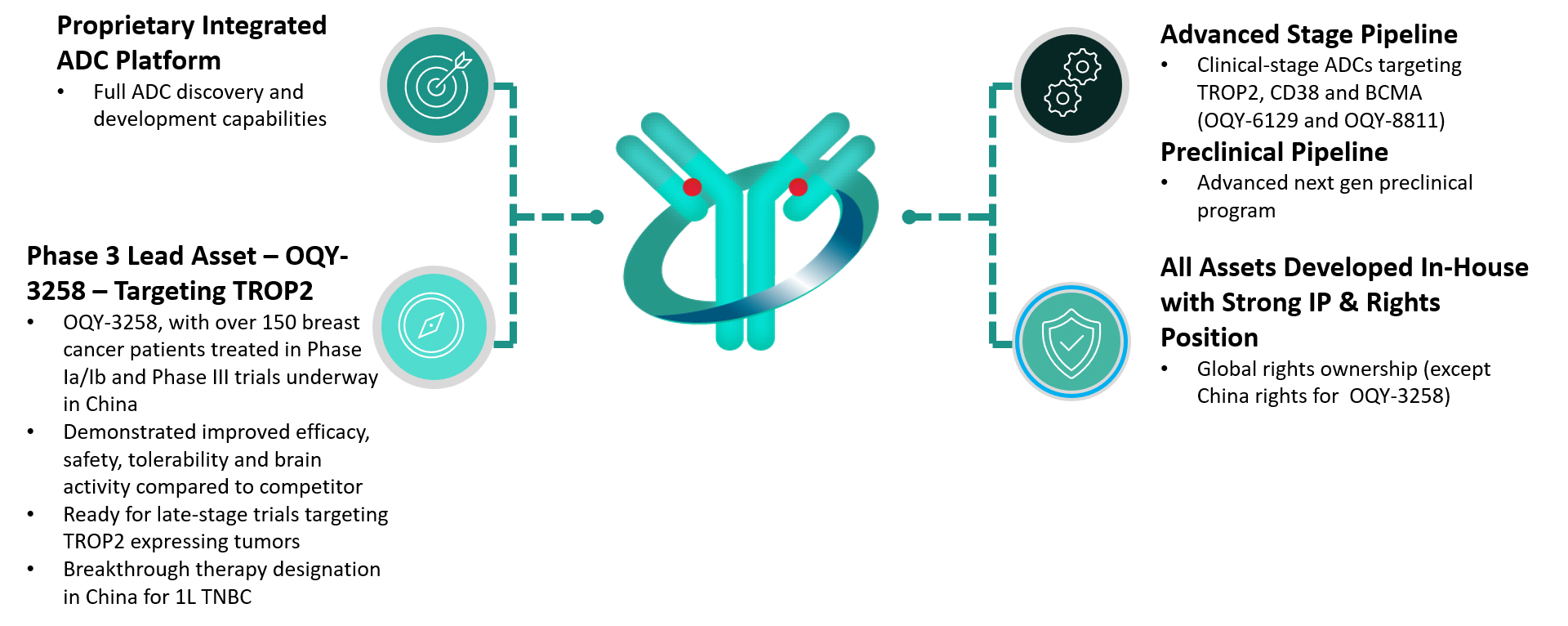
Oqory is a clinical-stage company developing advanced antibody-drug conjugates for the treatment of multiple oncology indications. We have a robust pipeline of clinical candidates and a complete platform for ADC discovery and development.
